Discovery of Benzimidazole–Quinolone Hybrids as New Cleaving Agents toward Drug‐Resistant Pseudomonas aeruginosa DNA
Ya‐Nan Wang; Rammohan R. Yadav Bheemanaboina; Wei‐Wei Gao; Jie Kang; Gui‐Xin Cai; Cheng‐He Zhou
Index: 10.1002/cmdc.201700739
Full Text: HTML
Abstract
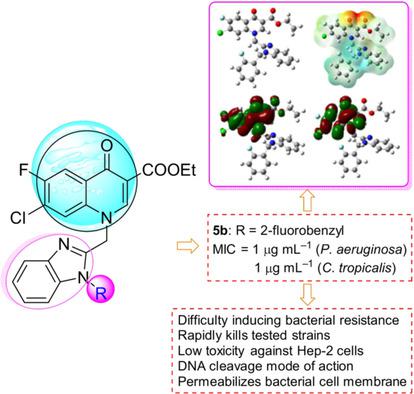
A series of benzimidazole–quinolone hybrids as new potential antimicrobial agents were designed and synthesized. Bioactive assays indicated that some of the prepared compounds exhibited potent antibacterial and antifungal activities. Notably, 2‐fluorobenzyl derivative 5 b (ethyl 7‐chloro‐6‐fluoro‐1‐[[1‐[(2‐fluorophenyl)methyl]benzimidazol‐2‐yl]methyl]‐4‐oxo‐quinoline‐3‐carboxylate) showed remarkable antimicrobial activity against resistant Pseudomonas aeruginosa and Candida tropicalis isolated from infected patients. Active molecule 5 b could not only rapidly kill the tested strains, but also exhibit low toxicity toward Hep‐2 cells. It was more difficult to trigger the development of bacterial resistance of P. aeruginosa against 5 b than that against norfloxacin. Molecular docking demonstrated that 5 b could effectively bind with topoisomerase IV–DNA complexes, and quantum chemical studies theoretically elucidated the good antimicrobial activity of compound 5 b. Preliminary experimental reaction mechanism exploration suggested that derivative 5 b could not intercalate into DNA isolated from drug‐resistant P. aeruginosa, but was able to cleave DNA effectively, which might further block DNA replication to exert powerful bioactivities. In addition, compound 5 b is a promising antibacterial agent with membrane disruption abilities.
|
Identifying Small‐Molecule Binding Sites for Epigenetic Prot...
2018-04-17 [10.1002/cmdc.201800030] |
|
Fluorinated GluN2B Receptor Antagonists with a 3‐Benzazepine...
2018-04-17 [10.1002/cmdc.201700819] |
|
Synthesis, Pharmacological Evaluation, and Docking Studies o...
2018-04-16 [10.1002/cmdc.201800152] |
|
Transthyretin Mimetics as Anti‐β‐Amyloid Agents: A Compariso...
2018-04-16 [10.1002/cmdc.201800031] |
|
Antibody Epitope of Human α‐Galactosidase A Revealed by Affi...
2018-04-16 [10.1002/cmdc.201800094] |