Aminoisoxazoles as Potent Inhibitors of Tryptophan 2,3-Dioxygenase 2 (TDO2)
Zhonghua Pei, Rohan Mendonca, Lewis Gazzard, Richard Pastor, Leanne Goon, Amy Gustafson, Erica VanderPorten, Georgia Hatzivassiliou, Kevin Dement, Robert Cass, Po-wai Yuen, Yamin Zhang, Guosheng Wu, Xingyu Lin, Yichin Liu, Benjamin D. Sellers
Index: 10.1021/acsmedchemlett.7b00427
Full Text: HTML
Abstract
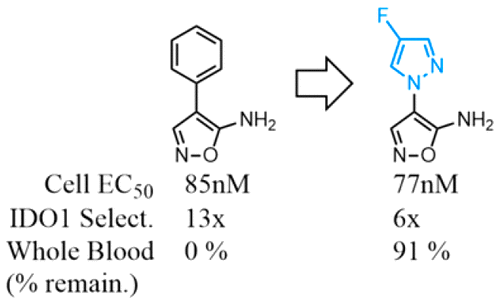
Tryptophan 2,3-dioxygenase 2 (TDO2) catalyzes the conversion of tryptophan to the immunosuppressive metabolite kynurenine. TDO2 overexpression has been observed in a number of cancers; therefore, TDO inhibition may be a useful therapeutic intervention for cancers. We identified an aminoisoxazole series as potent TDO2 inhibitors from a high-throughput screen (HTS). An extensive medicinal chemistry effort revealed that both the amino group and the isoxazole moiety are important for TDO2 inhibitory activity. Computational modeling yielded a binding hypothesis and provided insight into the observed structure–activity relationships. The optimized compound 21 is a potent TDO2 inhibitor with modest selectivity over indolamine 2,3-dioxygenase 1 (IDO1) and with improved human whole blood stability.
|
Discovery of MK-8282 as a Potent G-Protein-Coupled Receptor ...
2018-04-11 [10.1021/acsmedchemlett.8b00073] |
|
Academic Drug Development: The DRIVE Model
2018-04-11 [10.1021/acsmedchemlett.8b00124] |
|
SAR Studies of N-[2-(1H-Tetrazol-5-yl)phenyl]benzamide Deriv...
2018-04-10 [10.1021/acsmedchemlett.7b00510] |
|
Structure–Activity Relationships of Radioiodinated Benzoimid...
2018-04-10 [10.1021/acsmedchemlett.8b00092] |
|
Design, Synthesis, and X-ray of Selenides as New Class of Ag...
2018-04-10 [10.1021/acsmedchemlett.8b00076] |