Integration of Enhanced Sampling Methods with Saturation Transfer Difference Experiments to Identify Protein Druggable Pockets
Joana Magalhães, Giannamaria Annunziato, Nina Franko, Marco Pieroni, Barbara Campanini, Agostino Bruno, Gabriele Costantino
Index: 10.1021/acs.jcim.7b00733
Full Text: HTML
Abstract
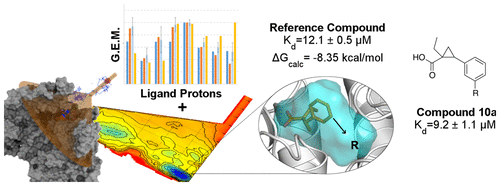
Saturation transfer difference (STD) is an NMR technique conventionally applied in drug discovery to identify ligand moieties relevant for binding to protein cavities. This is important to direct medicinal chemistry efforts in small-molecule optimization processes. However, STD does not provide any structural details about the ligand–target complex under investigation. Herein, we report the application of a new integrated approach, which combines enhanced sampling methods with STD experiments, for the characterization of ligand–target complexes that are instrumental for drug design purposes. As an example, we have studied the interaction between StOASS-A, a potential antibacterial target, and an inhibitor previously reported. This approach allowed us to consider the ligand–target complex from a dynamic point of view, revealing the presence of an accessory subpocket which can be exploited to design novel StOASS-A inhibitors. As a proof of concept, a small library of derivatives was designed and evaluated in vitro, displaying the expected activity.
|
Holistic Approach to Partial Covalent Interactions in Protei...
2018-04-19 [10.1021/acs.jcim.7b00398] |
|
Force Field Benchmark of Amino Acids: I. Hydration and Diffu...
2018-04-18 [10.1021/acs.jcim.8b00026] |
|
Role of Molecular Interactions and Protein Rearrangement in ...
2018-04-16 [10.1021/acs.jcim.7b00640] |
|
Peptidic Macrocycles - Conformational Sampling and Thermodyn...
2018-04-13 [10.1021/acs.jcim.8b00097] |
|
ReFlex3D: Refined Flexible Alignment of Molecules Using Shap...
2018-04-13 [10.1021/acs.jcim.7b00618] |