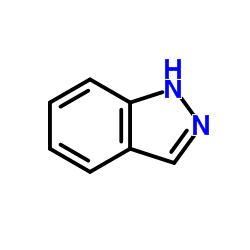
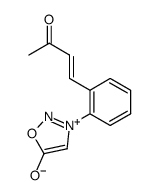
|
~43% |
|
~20% |
|
~36% |
|
~% |
|
~56% |
|
~13% |
|
~40% |
|
~28% |
|
~57% |
|
~28% |

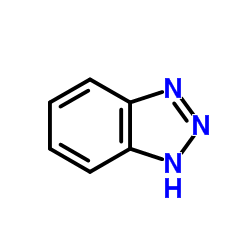
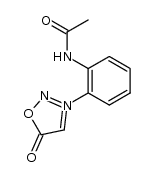
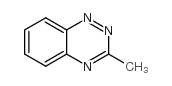

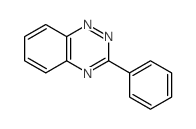

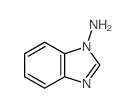
![3-[2-(Trifluoroacetamido)phenyl]sydnone Structure](https://image.chemsrc.com/caspic/340/104273-22-5.png)


![3-[2-[(Hydroximino)methyl]phenyl]sydnone Structure](https://image.chemsrc.com/caspic/201/106776-33-4.png)
![3-[2-[1-(Hydroximino)ethyl]phenyl]sydnone Structure](https://image.chemsrc.com/caspic/239/106776-32-3.png)