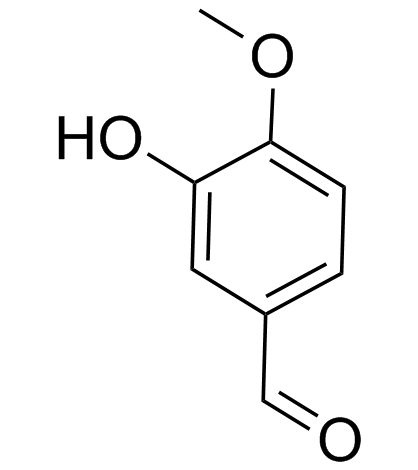
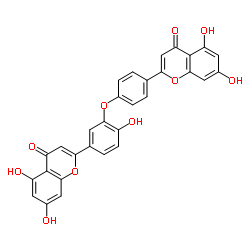
|
~% |
|
~% |
|
~% |
|
~58% |
|
~% |
|
~% |
|
~% |

![2-[3-[4-(5,7-dimethoxy-4-oxo-chromen-2-yl)phenoxy]-4-methoxy-phenyl]-5,7-dimethoxy-chromen-4-one Structure](https://image.chemsrc.com/caspic/146/49619-88-7.png)