|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
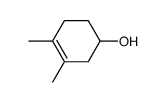
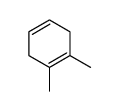
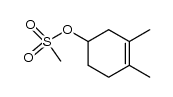
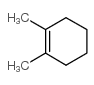
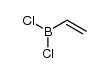
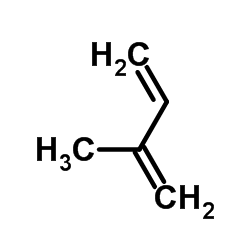
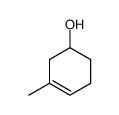
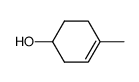

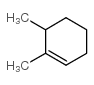

![Bicyclo[2.2.1]hept-5-en-2-ol,(1R,2R,4R)-rel Structure](https://image.chemsrc.com/caspic/028/694-97-3.png)