|
~99% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~59% |
|
~% |
|
~% |
|
~% |
|
~99% |

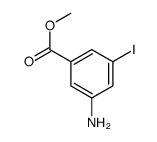
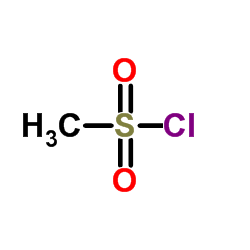
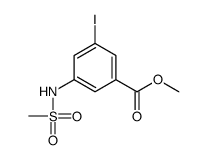
![methyl 3-iodo-5-[methyl(methylsulfonyl)amino]benzoate Structure](https://image.chemsrc.com/caspic/154/847157-48-6.png)
![methyl 3-allyl-5-[methyl(methylsulfonyl)amino]benzoate Structure](https://image.chemsrc.com/caspic/116/847157-49-7.png)
![3-[methyl(methylsulfonyl)amino]-5-prop-2-enylbenzoic acid Structure](https://image.chemsrc.com/caspic/251/847157-50-0.png)
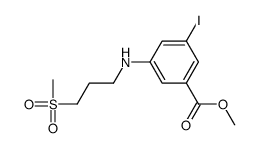
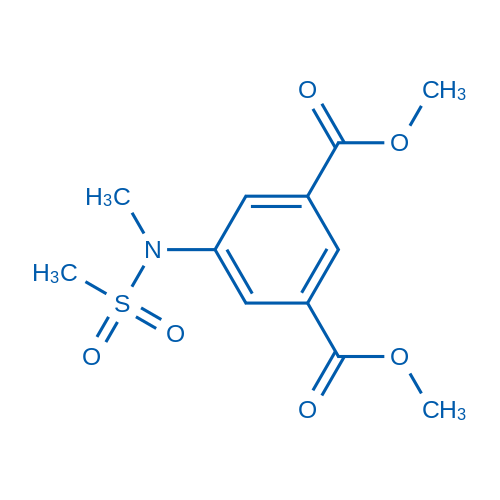
![5-[methyl(methylsulfonyl)amino]benzene-1,3-dicarboxylic acid Structure](https://image.chemsrc.com/caspic/155/913626-11-6.png)