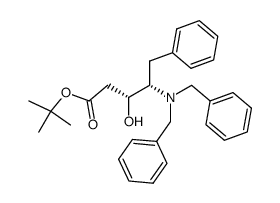
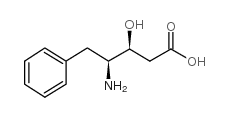
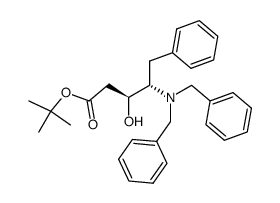
|
~96% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |

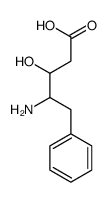
![2S-[bis(phenylmethyl)amino]benzenepropanaldehyde Structure](https://image.chemsrc.com/caspic/122/123054-12-6.png)