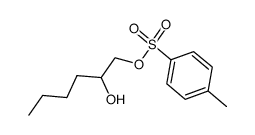
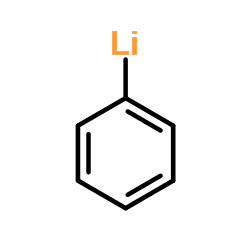
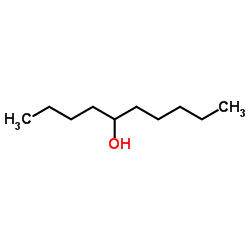
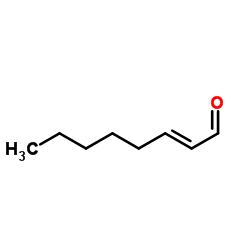
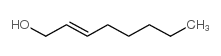
|
~91% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
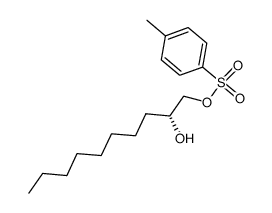
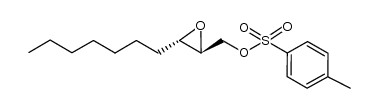
~92% |

![[(2R,3R)-3-dodecyloxiran-2-yl]methanol Structure](https://image.chemsrc.com/caspic/427/165880-19-3.png)


![(+/-)-2-[p-toluenesulfonyloxymethyl]-(2S*,3S*)-3-propyloxirane Structure](https://image.chemsrc.com/caspic/051/114395-17-4.png)