|
~85% |
|
~45% |
|
~% |
|
~% |
|
~63% |
|
~% |
|
~% |
![4-Chloro-1H-pyrrolo[2,3-b]pyridine Structure](https://image.chemsrc.com/caspic/400/55052-28-3.png)
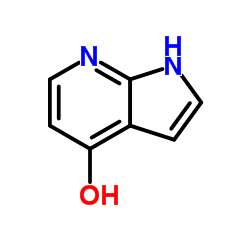
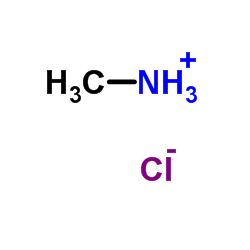
![1H-Pyrrolo[3,2-c]pyridin-4-amine,1-methyl-(9CI) Structure](https://image.chemsrc.com/caspic/287/102839-56-5.png)
![4-Methoxy-1H-pyrrolo[2,3-b]pyridine Structure](https://image.chemsrc.com/caspic/034/122379-63-9.png)
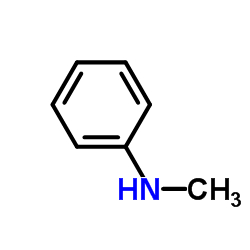
![N-methyl-N-phenyl-1H-pyrrolo[2,3-b]pyridin-4-amine Structure](https://image.chemsrc.com/caspic/486/122379-64-0.png)