|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

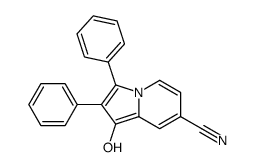
![7-[2-(3-oxo-1,2-diphenylindolizin-7-ylidene)ethylidene]-1,2-diphenylindolizin-3-one Structure](https://image.chemsrc.com/caspic/330/86193-55-7.png)
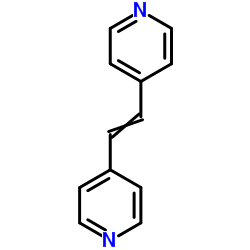
![7-[2-(1-oxo-2,3-diphenylindolizin-7-ylidene)ethylidene]-2,3-diphenylindolizin-1-one Structure](https://image.chemsrc.com/caspic/254/86193-57-9.png)
