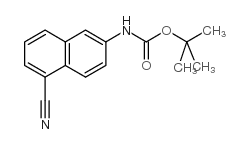
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
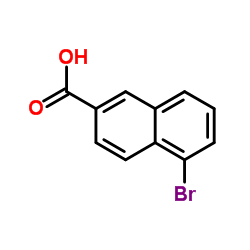
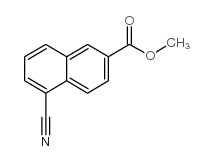
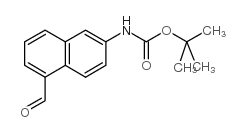
![TERT-BUTYL [5-(HYDROXYMETHYL)-2-NAPHTHYL]CARBAMATE Structure](https://image.chemsrc.com/caspic/233/685902-89-0.png)
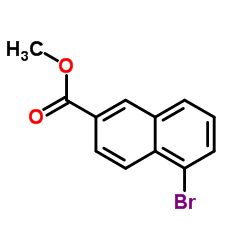
![(Z)-[(dimethoxyphosphinyl)oxy]-1,2-dimethylethylene Structure](https://image.chemsrc.com/caspic/068/5043-32-3.png)