|
~97% |
|
~98% |
|
~% |
|
~% |

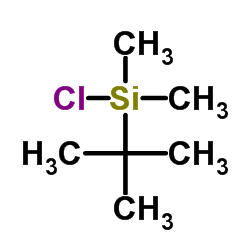
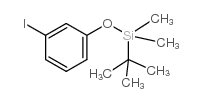
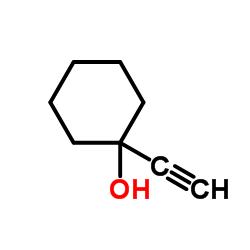
![1-[2-[3-[tert-butyl(dimethyl)silyl]oxyphenyl]ethynyl]cyclohexan-1-ol Structure](https://image.chemsrc.com/caspic/200/188957-23-5.png)