|
~77% |
|
~71% |
|
~% |
|
~% |
|
~% |
|
~% |
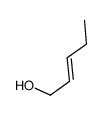
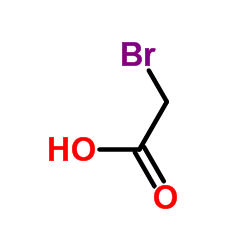
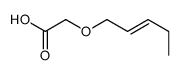

![(2R,3S)-1-((3aS,8aR)-2,2-dimethyl-8,8a-dihydro-2H-indeno[1,2-d]oxazol-3(3aH)-yl)-3-ethyl-2-hydroxypent-4-en-1-one Structure](https://image.chemsrc.com/caspic/418/189690-79-7.png)


![1-((3aS,8aR)-2,2-dimethyl-8,8a-dihydro-2H-indeno[1,2-d]oxazol-3(3aH)-yl)-2-((E)-pent-2-en-1-yloxy)ethanone Structure](https://image.chemsrc.com/caspic/456/189690-78-6.png)