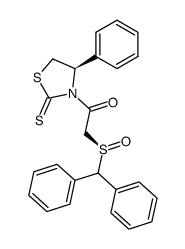
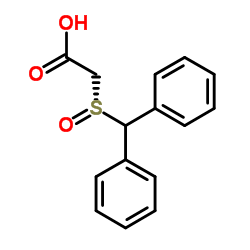
|
~89% |
|
~% |
|
~89% |
|
~% |
|
~96% |
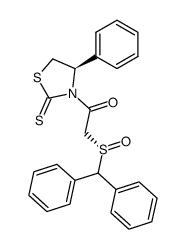
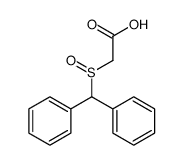
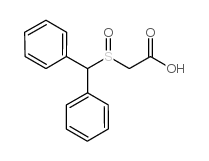

![ethyl 2-[(S)-benzhydrylsulfinyl]acetate Structure](https://image.chemsrc.com/caspic/139/827604-05-7.png)