| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
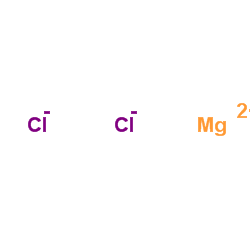 |
Magnesium choride
CAS:7786-30-3 |
|
 |
L-(+)-Lysine monohydrochloride
CAS:657-27-2 |
|
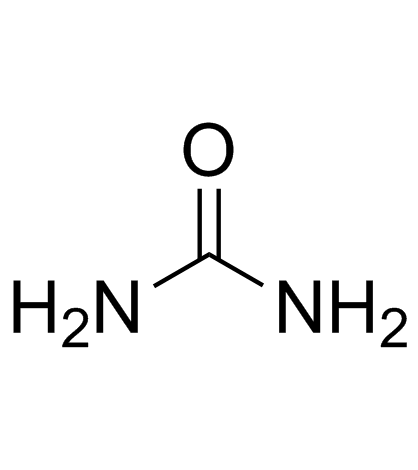 |
Urea
CAS:57-13-6 |
|
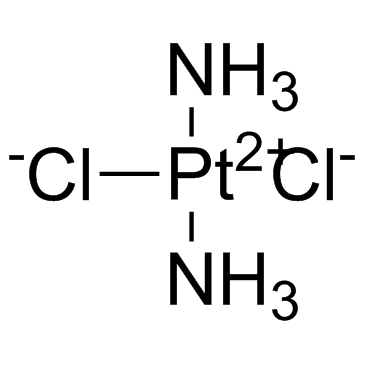 |
Cisplatin
CAS:15663-27-1 |
|
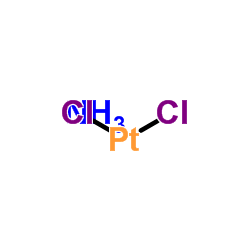 |
trans-Dichlorodiamineplatinum(II)
CAS:14913-33-8 |
|
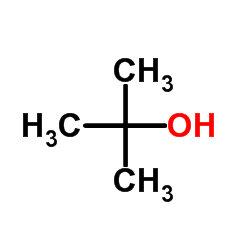 |
tert-Butanol
CAS:75-65-0 |
|
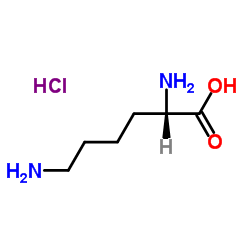 |
L-Lysine hydrochloride
CAS:10098-89-2 |
|
 |
MES
CAS:4432-31-9 |
|
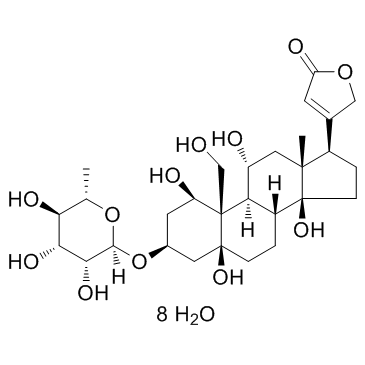 |
Ouabain Octahydrate
CAS:11018-89-6 |