| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
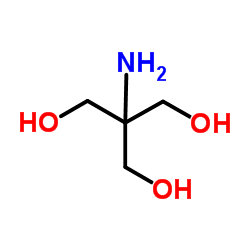 |
Trometamol
CAS:77-86-1 |
|
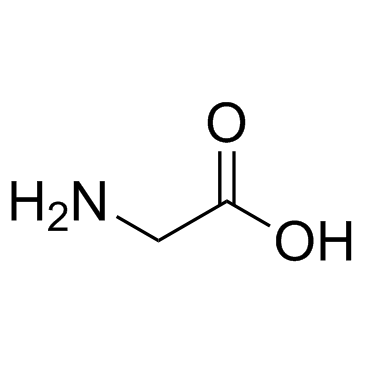 |
Glycine
CAS:56-40-6 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
methylamine
CAS:74-89-5 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
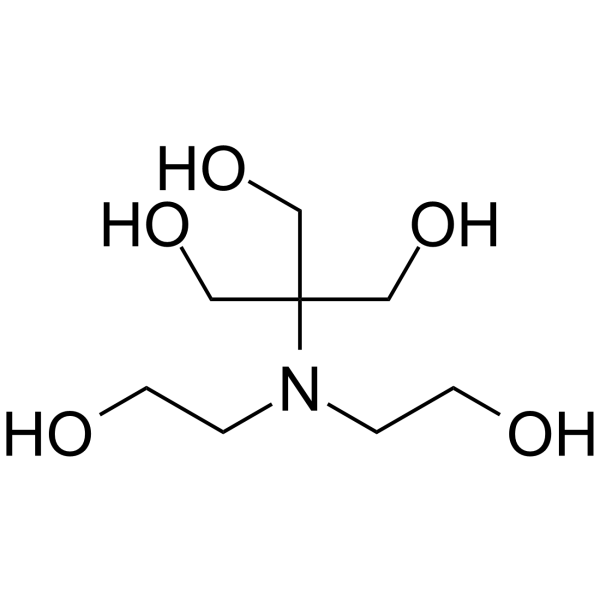 |
Bis-tris methane
CAS:6976-37-0 |