| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
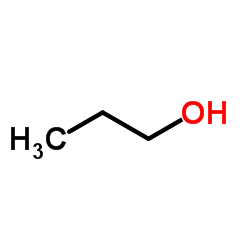 |
1-Propanol
CAS:71-23-8 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
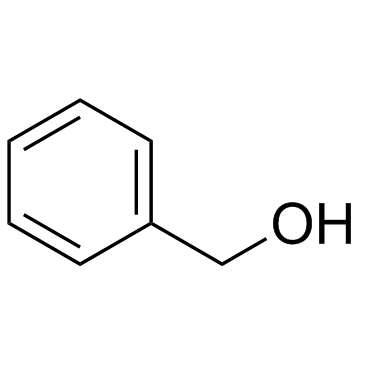 |
Benzyl alcohol
CAS:100-51-6 |
|
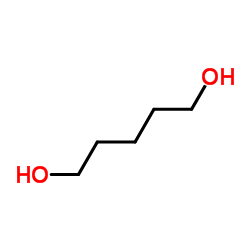 |
1,5-Pentanediol
CAS:111-29-5 |
|
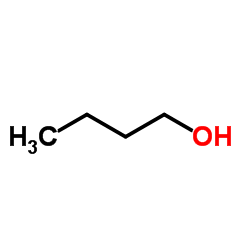 |
Butanol
CAS:71-36-3 |
|
 |
Decan-1-ol
CAS:112-30-1 |
|
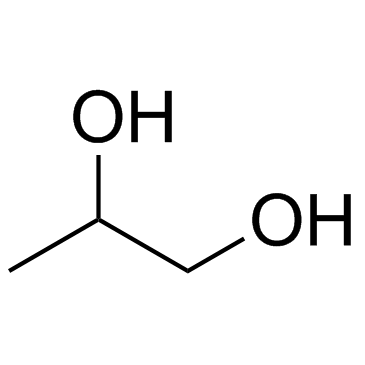 |
Propylene Glycol
CAS:57-55-6 |
|
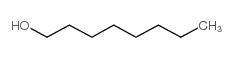 |
1-Octanol
CAS:111-87-5 |