| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Ethanol
CAS:64-17-5 |
|
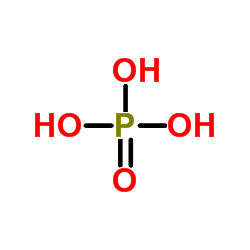 |
Phosphoric acid
CAS:7664-38-2 |
|
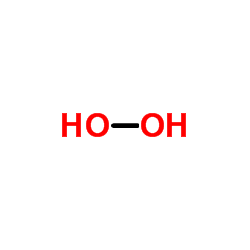 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
 |
Methanol
CAS:67-56-1 |
|
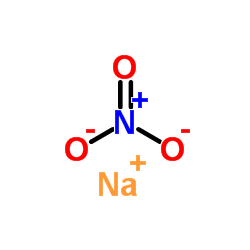 |
sodium nitrate
CAS:7631-99-4 |
|
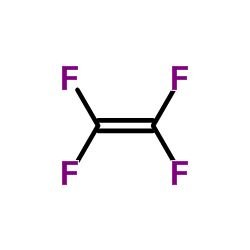 |
poly(tetrafluoroethylene)
CAS:9002-84-0 |
|
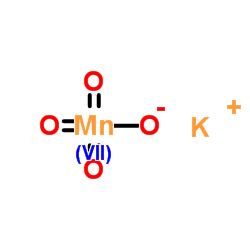 |
Potassium permanganate
CAS:7722-64-7 |