| Structure | Name/CAS No. | Articles |
|---|---|---|
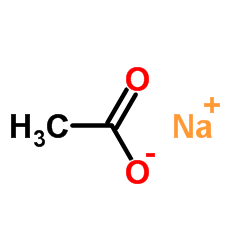 |
Sodium acetate
CAS:127-09-3 |
|
 |
Chloroform
CAS:67-66-3 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Methanol
CAS:67-56-1 |
|
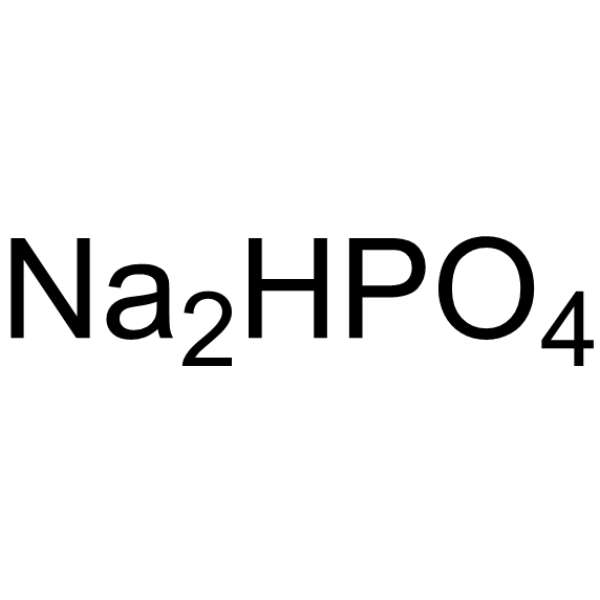 |
Disodium hydrogenorthophosphate
CAS:7558-79-4 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
Formic Acid
CAS:64-18-6 |
|
 |
carbon black
CAS:1333-86-4 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |