| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodiumborohydride
CAS:16940-66-2 |
|
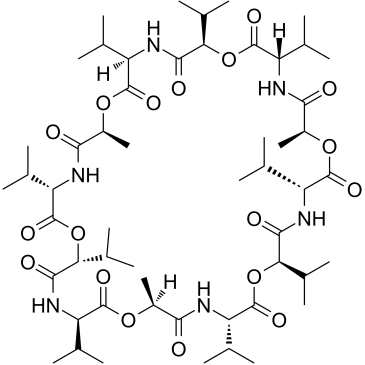 |
Valinomycin
CAS:2001-95-8 |
|
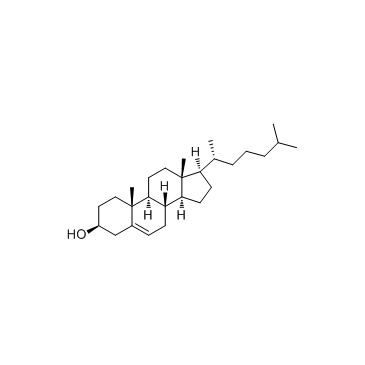 |
cholesterol
CAS:57-88-5 |
|
 |
Ergosterol
CAS:57-87-4 |
|
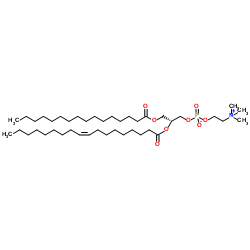 |
1-Palmitoyl-2-oleoyl-sn-glycero-3-PC
CAS:26853-31-6 |
|
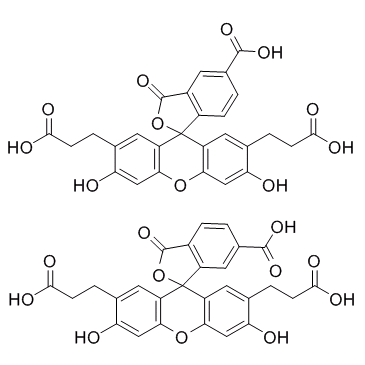 |
BCECF
CAS:85138-49-4 |
|
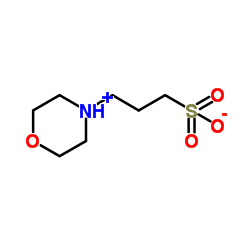 |
MOPS
CAS:1132-61-2 |
|
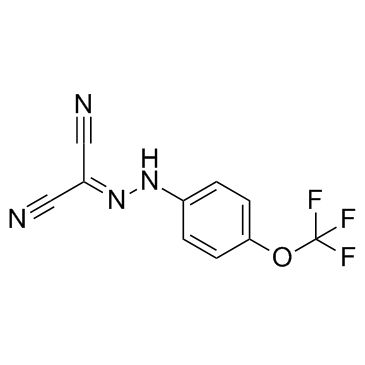 |
FCCP
CAS:370-86-5 |