| Structure | Name/CAS No. | Articles |
|---|---|---|
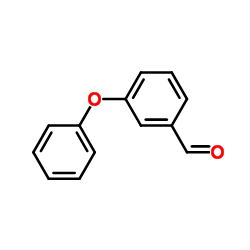 |
3-Phenoxybenzaldehyde
CAS:39515-51-0 |
|
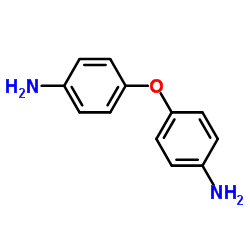 |
4,4'-Oxydianiline
CAS:101-80-4 |
|
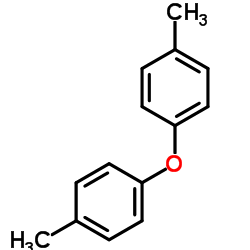 |
bis(4-methylphenyl) ether
CAS:1579-40-4 |