Fluorescence quenching of 4-tert-octylphenol by room temperature ionic liquids and its application.
Huili Wang, Jingwen Mao, Ailian Duan, Baoguang Che, Wenwei Wang, Meiping Ma, Xuedong Wang
Index: J. Fluoresc. 23(2) , 323-31, (2013)
Full Text: HTML
Abstract
The interactions between room temperature ionic liquids (RTILs) and weak fluorescent chemicals still remain unclear, which hinders the complete and efficient utilization of these "green" solvents in fluorescent analyses of organic chemicals. Herein, we reported the effects of four RTILs, [C8MIM]BF4, [C14MIM]BF4, [C8MIM]PF6 and [C14MIM]PF6, on fluorescence behavior of 4-tert-octylphenol (4-t-OP). In the fortified concentration range of 0.2-1.0 mM, the quenching effects were increased with increasing concentrations of RTILs. However, no obvious variation of peak shape of 4-t-OP was observed in the quenching process, suggesting no formation of ground-state complex between fluorophores in 4-t-OP and quencher (ionic liquids). As for anion effect, the fluorescence quenching efficiency of 4-t-OP by BF4(-) was greater than PF6(-), but the carbon chain length on the imidazolium ring had no significant relationship with fluorescence intensity of 4-t-OP. Both Ksv values (>1.0 × 10(3) L/mol.s) and the different temperature effects demonstrated that the quenching of 4-t-OP by four RTILs was the presence of dynamic and static quenching mechanism. The FI of dansyl chloride within [C8MIM]BF4 increased nearly 5-fold as compared to the control, showing a sensitizing effect on the strong fluorescent chemicals, while a quenching effect on 4-t-OP belonging to weak fluorescent chemicals. The fluorescence-enhanced amplitude of dansyl chloride in [C8MIM]PF6 was greater than [C8MIM]BF4. The fluorescence quenching of 4-t-OP by [C8MIM]PF6 did not belong to FRET phenomenon because of no overlap of emission spectrum of 4-t-OP and absorption spectrum of [C8MIM]PF6. When 0.6 mM [C8MIM]PF6 in acetonitrile was used as the solvent, the detection limit of 4-t-OP was 3.7 μg/L, and the linearity range was 0.01-0.8 mg/L (R(2) = 0.9990). In summary, these results provide a theoretical foundation for the application of RTILs in weak fluorescent chemicals.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
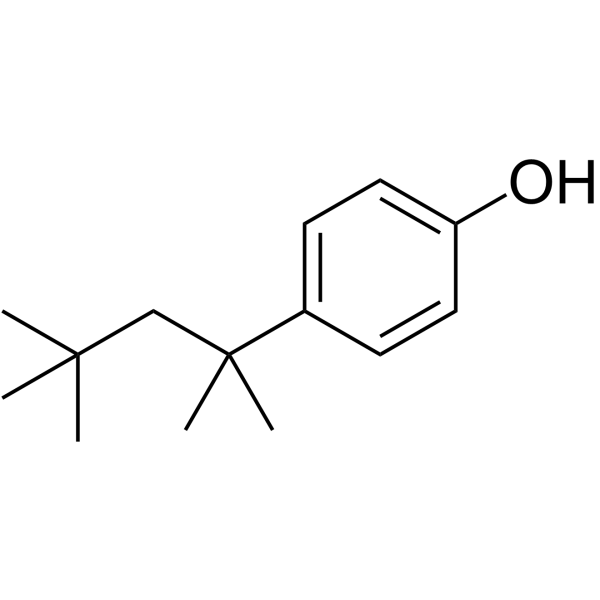 |
4-tert-Octylphenol
CAS:140-66-9 |
C14H22O |
|
Effects of Endocrine Disruptor Compounds, Alone or in Combin...
2015-01-01 [PLoS ONE 10 , e0131428, (2015)] |
|
Liquid chromatographic-tandem mass spectrometric method for ...
2014-10-03 [J. Chromatogr. A. 1362 , 75-88, (2014)] |
|
Occurrence and spatial distribution of EDCs and related comp...
2015-01-15 [Sci. Total Environ. 503-504 , 69-86, (2015)] |
|
Differential accumulation levels in the brain of rats expose...
2011-01-01 [Environ. Toxicol. Pharmacol. 31(1) , 198-204, (2011)] |
|
Photocatalytic degradation of 4-tert-octylphenol in a spiral...
2012-01-01 [J. Environ. Sci. (China) 24(9) , 1679-85, (2012)] |