5-Selenization of salicylic acid derivatives yielded isoform-specific 5-lipoxygenase inhibitors.
Sun-Chol Yu, Hartmut Kuhn, Constantin-Gabriel Daniliuc, Igor Ivanov, Peter G Jones, Wolf-Walther du Mont
Index: Org. Biomol. Chem. 8(4) , 828-34, (2010)
Full Text: HTML
Abstract
Low molecular weight seleno-organic compounds exhibit glutathione peroxidase (GPx)-like activity; the well-known compound ebselen is being used in clinical trials as a stroke medication. Here, we describe the facile one-step synthesis of novel 5-selenized salicylic acid derivatives using selenium tetrachloride. The products were analyzed by spectroscopic studies including (77)Se-NMR and some were subjected to X-ray structure determination. Several products were identified as selective inhibitors of the pro-inflammatory 5-lipoxygenase (LOX) but had little effect on the catalytic activity of 12/15-LOX, which has been implicated in the synthesis of anti-inflammatory mediators. Such isoform-specificity (specificity coefficient >120) has not been reported before for any seleno-organic compound. In addition, synthesis products exhibited GPx-like activity, which was higher than that of ebselen for some derivatives.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
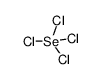 |
Selenium(IV) chloride (-8 mesh)
CAS:10026-03-6 |
Cl4Se |
|
Lipocalin-type prostaglandin D synthase produces prostagland...
2006-11-21 [Proc. Natl. Acad. Sci. U. S. A. 103(47) , 17949-54, (2006)] |
|
Prostaglandin D synthase in the prenatal ovine brain and eff...
2002-07-01 [J. Neurosci. 22(13) , 5679-86, (2002)] |
|
Inhibition of sleep in rats by inorganic selenium compounds,...
1991-10-15 [Proc. Natl. Acad. Sci. U. S. A. 88(20) , 9046-50, (1991)] |
|
Inhibition of rat brain prostaglandin D synthase by inorgani...
1991-08-15 [Arch. Biochem. Biophys. 289(1) , 161-6, (1991)] |
|
Retrograde tracing of zinc-containing neurons by selenide io...
1992-04-01 [J. Histochem. Cytochem. 40(4) , 575-9, (1992)] |