A closer view of the conformation of the Lac repressor bound to operator.
C E Bell, M Lewis
Index: Nat. Struct. Biol. 7(3) , 209-14, (2000)
Full Text: HTML
Abstract
Crystal structures of the Lac repressor, with and without isopropyithiogalactoside (IPTG), and the repressor bound to operator have provided a model for how the binding of the inducer reduces the affinity of the repressor for the operator. However, because of the low resolution of the operator-bound structure (4.8 A), the model for the allosteric transition was presented in terms of structural elements rather than in terms of side chain interactions. Here we have constructed a dimeric Lac repressor and determined its structure at 2.6 A resolution in complex with a symmetric operator and the anti-inducer orthonitrophenylfucoside (ONPF). The structure enables the induced (IPTG-bound) and repressed (operator-bound) conformations of the repressor to be compared in atomic detail. An extensive network of interactions between the DNA-binding and core domains of the repressor suggests a possible mechanism for the allosteric transition.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
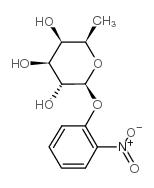 |
2-nitrophenyl β-D-fucoside
CAS:1154-94-5 |
C12H15NO7 |
|
Thermal denaturation of the core protein of lac repressor.
1985-07-16 [Biochemistry 24(15) , 3842-6, (1985)] |
|
Escherichia coli lac repressor-lac operator interaction and ...
1997-01-10 [J. Mol. Biol. 265(1) , 1-7, (1997)] |
|
Using networks to identify fine structural differences betwe...
2004-08-31 [Biochemistry 43(34) , 10886-95, (2004)] |
|
Altering residues N125 and D149 impacts sugar effector bindi...
2011-10-25 [Biochemistry 50(42) , 9002-13, (2011)] |
|
Ligand interactions with lactose repressor protein and the r...
2007-03-01 [Biophys. Chem. 126(1-3) , 94-105, (2007)] |