A sensitive high-throughput HPLC assay for simultaneous determination of everolimus and clobetasol propionate.
Marika Kamberi, Katherine Fu, Jianmin Lu, G Mike Chemaly, Debra Feder
Index: J. Chromatogr. Sci. 46(1) , 23-9, (2008)
Full Text: HTML
Abstract
A novel sensitive high-throughput high-performance liquid chromatography assay is developed and validated for the simultaneous determination of everolimus and clobetasol propionate in pharmaceutical formulations. The chromatographic separation is achieved on a Zorbax Eclipse XDB-C18 reversed-phase column using a gradient elution, with solvent A: ammonium acetate (pH 6.8; 0.01 M) and solvent B: acetonitrile. The mean recovery ranges from 95.1% to 100.0% for clobetasol propionate and from 97.9% to 103.7% for everolimus. The limit of quantitation for each analyte is 0.02 microg/mL. The percent relative standard deviations are less than 3% for intra- and inter-day analyses. The proposed method can be used for the routine quality control of everolimus and clobetasol propionate in complex pharmaceutical formulations, especially the drug-delivery systems with a low total drug-load.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
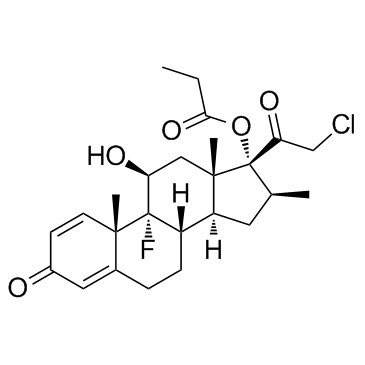 |
Clobetasol propionate
CAS:25122-46-7 |
C25H32ClFO5 |
|
Curcumin relieves TPA-induced Th1 inflammation in K14-VEGF t...
2015-04-01 [Int. Immunopharmacol. 25(2) , 235-41, (2015)] |
|
Topical steroids for chronic wounds displaying abnormal infl...
2013-05-01 [Ann. R. Coll. Surg. Engl. 95(4) , 291-6, (2013)] |
|
Impact of clobetasol propionate 0.05% spray on health-relate...
2012-11-01 [Journal. of. Drugs in. Dermatology. 11(11) , 1348-54, (2012)] |
|
Clobetasol propionate spray 0.05% for the treatment of moder...
2012-02-01 [Cutis. 89(2) , 89-94, (2012)] |
|
Influence of the type of vegetable oil on the drug release p...
2014-11-01 [Pharm. Dev. Technol. 19(7) , 789-98, (2014)] |