| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
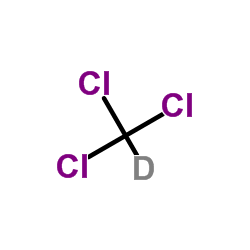 |
chloroform-d
CAS:865-49-6 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
 |
Butyryl chloride
CAS:141-75-3 |
|
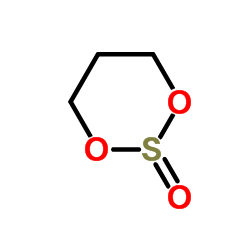 |
1,3,2-Dioxathiane 2-oxide
CAS:4176-55-0 |