| Structure | Name/CAS No. | Articles |
|---|---|---|
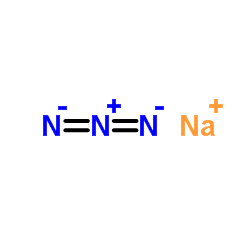 |
Sodium azide
CAS:26628-22-8 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Aqueous ammonia
CAS:1336-21-6 |
|
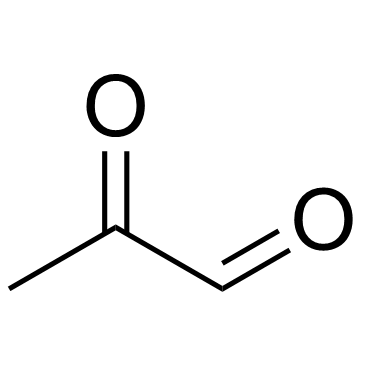 |
Methylglyoxal
CAS:78-98-8 |
|
 |
Formic Acid
CAS:64-18-6 |
|
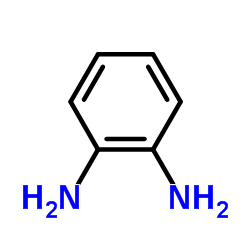 |
o-Phenylenediamine
CAS:95-54-5 |
|
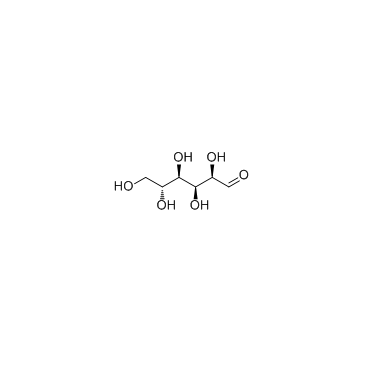 |
D-(+)-Glucose
CAS:50-99-7 |
|
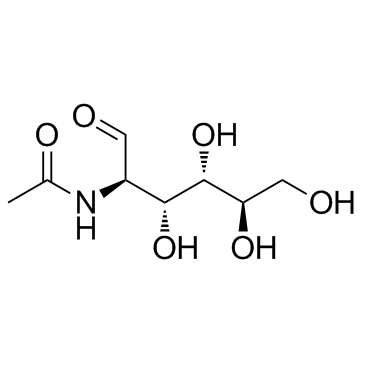 |
N-acetyl-α-D-glucosamine
CAS:7512-17-6 |
|
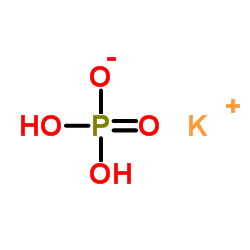 |
Monopotassium phosphate
CAS:7778-77-0 |