| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sucrose
CAS:57-50-1 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
L-(+)-Lysine monohydrochloride
CAS:657-27-2 |
|
 |
vinyl acetate
CAS:108-05-4 |
|
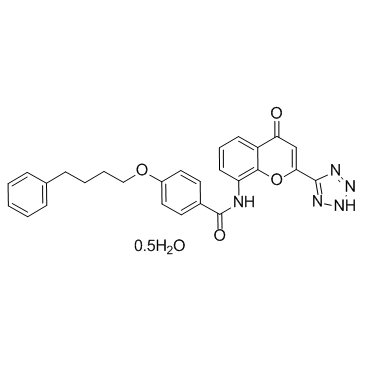 |
Pranlukast hemihydrate
CAS:150821-03-7 |