| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
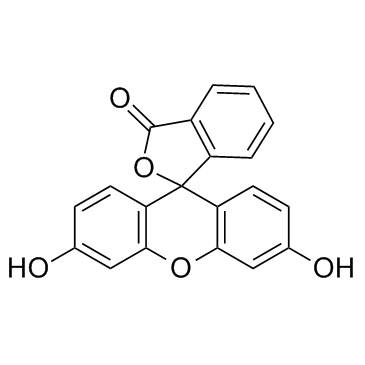 |
Fluorescein
CAS:2321-07-5 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
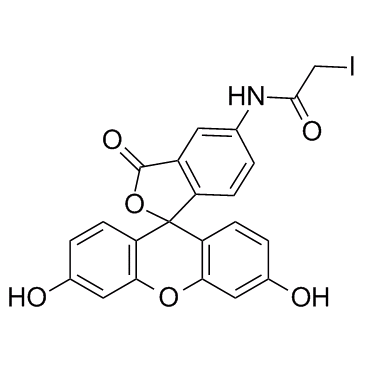 |
5-(Iodoacetamido)fluorescein
CAS:63368-54-7 |
|
 |
DIEA
CAS:7087-68-5 |