Synthesis of 6-aminouracils and pyrrolo[2,3-d]pyrimidine-2,4-diones and their inhibitory effect on thymidine phosphorylase.
K Hirota, M Sawada, H Sajiki, M Sako
Index: Nucleic Acids Symp. Ser. (37) , 59-60, (1997)
Full Text: HTML
Abstract
Inhibitors of thymidine phosphorylase (dThdPase) are expected to suppress the growth and metastasis of tumor cells by inhibition of angiogenesis and were designed by utilizing the three dimensional structure of the enzyme. 5-Substituted 6-aminouracils (5) and 7-substituted pyrrolo[2,3-d]pyrimidine-2,4-diones (6) were synthesized and tested for inhibition of human placenta thymidine phosphorylase. 5-Bromo-6-aminouracil (5d), 5-cyano-6-[3-(methylamino)propyl]-uracil (5e), and 7-(2-aminoethyl)-pyrrolo[2,3-d]pyrimidine-2,4-dione (6c) inhibited dThdPase with IC50s of 7.6, 3.8 and 44.0 microM, respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
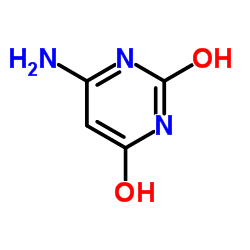 |
6-Aminouracil
CAS:873-83-6 |
C4H5N3O2 |
|
Thymidine phosphorylase inhibitors: recent developments and ...
2005-12-01 [Mini Rev. Med. Chem. 5(12) , 1113-23, (2005)] |
|
Synthesis, chemical and pharmacological properties of some 2...
1996-01-01 [Acta Pol. Pharm. 53(1) , 39-46, (1996)] |
|
Cocrystals of 5-fluorocytosine. I. Coformers with fixed hydr...
2012-08-01 [Acta Crystallogr. B 68(Pt 4) , 431-43, (2012)] |
|
6-Anilinouracil-based inhibitors of Bacillus subtilis DNA po...
1999-06-03 [J. Med. Chem. 42(11) , 2035-40, (1999)] |
|
Simultaneous determination of uric acid metabolites allantoi...
2009-01-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 877(1-2) , 65-70, (2009)] |