| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
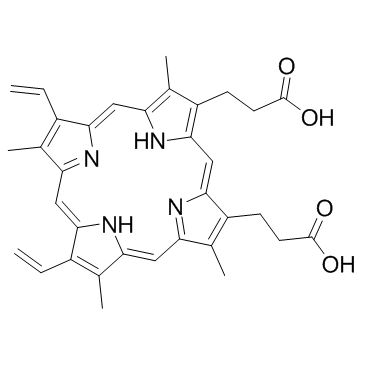 |
Protoporphyrin IX
CAS:553-12-8 |
|
 |
Cobalt chloride
CAS:7646-79-9 |