| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
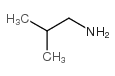 |
Isobutylamine
CAS:78-81-9 |
|
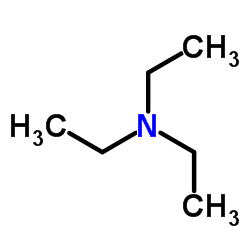 |
Triethylamine
CAS:121-44-8 |