Use of isotope effects to characterize intermediates in mechanism-based inactivation of dopamine beta-monooxygenase by beta-chlorophenethylamine.
M J Bossard, J P Klinman
Index: J. Biol. Chem. 265(10) , 5640-7, (1990)
Full Text: HTML
Abstract
A mechanism for beta-chlorophenethylamine inhibition of dopamine beta-monooxygenase has been postulated in which bound alpha-aminoacetophenone is generated followed by an intramolecular redox reaction to yield a ketone-derived radical cation as the inhibitory species (Mangold, J.B., and Klinman, J.P. (1984) J. Biol. Chem. 259, 7772-7779). Based on the assumption that the ketone radical is the inhibitory intermediate, an analogous system was predicted and verified (Bossard, M.J., and Klinman, J.P. (1986) J. Biol. Chem. 261, 16421-16427). In the present study, the role of alpha-aminoacetophenone as the proposed intermediate in the inactivation by beta-chlorophenethylamine was examined in greater detail. From the interdependence of tyramine and alpha-aminoacetophenone concentrations, ketone inactivation is concluded to occur at the substrate site as opposed to potential binding at the reductant-binding site. Using beta-[2-1H]- and beta-[2-2H]chlorophenethylamine, the magnitude of the deuterium isotope effect on inactivation under second-order conditions has been found to be identical to that observed under catalytic turnover, D(kappa inact/Ki) = D(kappa cat/Km) = 6-7. By contrast, the isotope effect on inactivation under conditions of substrate and oxygen saturation, D kappa inact = 2, is 3-fold smaller than that seen on catalytic turnover, D kappa cat = 6. This reduced isotope effect for inactivation is attributed to a normal isotope effect on substrate hydroxylation followed by an inverse isotope effect on the partitioning of the enol of alpha-aminoacetophenone between oxidation to a radical cation versus protonation to regenerate ketone. These findings are unusual in that two isotopically sensitive steps are present in the inactivation pathway whereas only one is observable in turnover.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
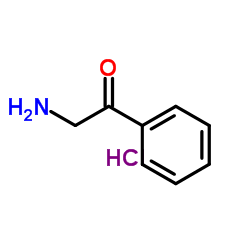 |
2-Amino-1-phenylethanone hydrochloride
CAS:5468-37-1 |
C8H10ClNO |
|
[Anorexigenic activity of various derivatives of alpha-amino...
1979-12-01 [Arch. Farmacol. Toxicol. 5(3) , 165-8, (1979)] |
|
Determination of cathinone, cathine and norephedrine in hair...
2003-04-23 [Forensic Sci. Int. 133(1-2) , 39-46, (2003)] |
|
Mechanism-based inactivation of dopamine beta-monooxygenase ...
1984-06-25 [J. Biol. Chem. 259(12) , 7772-9, (1984)] |
|
Dopamine beta-hydroxylase: activity and inhibition in the pr...
1982-01-05 [Biochemistry 21(1) , 67-75, (1982)] |
|
New entry of coupling reaction of phenacylamine derivatives ...
2002-03-01 [Chem. Pharm. Bull. 50(3) , 441-3, (2002)] |