| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Potassium bromide
CAS:7758-02-3 |
|
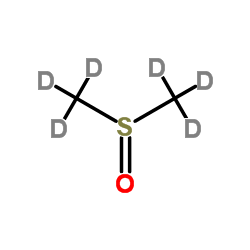 |
DIMETHYL SULFOXIDE-D6
CAS:2206-27-1 |
|
 |
Thymidine phosphorylase
CAS:9030-23-3 |