Solubility of Epiphany endodontic sealer prepared with resinous solvent.
Odival Mathias-Junior, Aline Evangelista Souza-Gabriel, Carlos Eduardo Saraiva Miranda, Jesus Djalma Pécora, Yara Teresinha Correa Silva-Sousa, Manoel Damião Sousa-Neto
Index: J. Endod. 35(5) , 715-8, (2009)
Full Text: HTML
Abstract
To evaluate in vitro the solubility of the Epiphany endodontic filling material Epiphany (Pentron Clinical Technologies, Wallingford, CT) prepared with its resinous solvent.The specimens were prepared in the following experimental conditions: (1) GI, epiphany without photoactivation; (2) GII, Epiphany prepared with resinous solvent without photoactivation; (3) GIII, Epiphany followed by photoactivation; and (4) GIV, Epiphany prepared with resinous solvent followed by photoactivation. Ten specimens of each group were obtained from Teflon molds with 80% reduction in volume of the specimen's dimensions based on American National Standard Institute/American Dental Association (ANSI/ADA) Specification No. 57. The samples were weighted and immersed in distilled water for 7 days. After this period, they were removed, dried, and weighed again. Solubility was calculated by using samples weight loss (%). The immersion liquid was evaluated through atomic absorption spectrometry.The sealers without photoactivation were statistically similar (p > 0.05) between themselves (GI = 6.93% and GII = 6.39%) and different from the uncured sealers, which were statistically different between themselves (p < 0.05) (GIII = 3.56% and GIV = 0.47%). Only the Epiphany sealer prepared with resinous solvent followed by photoactivation presented solubility values within ANSI/ADA requirements, liberating the following amounts of ions: 114.43 microg of Ca(2+)/mL, 2.4 microg of Mg(2+)/mL, 0.33 microg of Fe(2+)/mL, 0.11 microg of Zn(2+)/mL, 1.31 microg of Ni(2+)/mL, and 7.1 microg of Na(+)/mL.The association of resinous solvent to the Epiphany sealer followed by photoactivation resulted in a filling material with low solubility and expressive liberation of calcium ions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
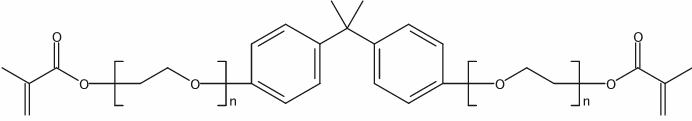 |
Bisphenol A ethoxylate dimethacrylate
CAS:41637-38-1 |
(C2H4O)n(C2H4O)nC23H24O4 |
|
Antibacterial dental composites with chlorhexidine and mesop...
2014-12-01 [J. Dent. Res. 93(12) , 1283-9, (2014)] |
|
Effects of light exposure time on composite resin hardness a...
2008-07-01 [J. Dent. 36(7) , 520-8, (2008)] |
|
Novel F-releasing composite with improved mechanical propert...
2009-01-01 [J. Dent. Res. 88(1) , 83-8, (2009)] |
|
Tissue engineering scaffolds based on photocured dimethacryl...
2006-06-01 [Biomacromolecules 7(6) , 1751-7, (2006)] |
|
A comparison of the wear resistance and hardness of indirect...
2001-04-01 [J. Prosthet. Dent. 85(4) , 386-95, (2001)] |