Trimethylphosphine binding to horse-heart and sperm-whale myoglobins. Kinetics, proton magnetic resonance assignment and nuclear Overhauser effect investigation of the heme pocket.
C Brunel, A Bondon, G Simonneaux
Index: Eur. J. Biochem. 214(2) , 405-14, (1993)
Full Text: HTML
Abstract
Two-dimensional nuclear magnetic resonance techniques have been used to assign resonances corresponding to the heme pocket and several other residues of horse heart and sperm whale myoglobins ligated by trimethylphosphine. The assignment procedure was based mainly on the nuclear Overhauser effect connectivities with the ligand and the heme substituents. For quantitative measurements of Overhauser effects, application of truncated driven techniques between a proton from distal residues and methyl groups from the ligand was used to determine internuclear distances. These new results have permitted us to map the heme pockets and to investigate the conformational differences in the heme pockets between horse heart and sperm whale myoglobins. The interproton distances between distal amino acid residues and trimethylphosphine were found to be longer in horse heart myoglobin relative to those in sperm whale myoglobin. This result suggests that the size of the heme pocket is larger in horse heart myoglobin. Association and dissociation rate constants were measured for trimethylphosphine binding to myoglobins. Both values were four times larger for horse heart myoglobin than those for sperm whale myoglobin. This observation confirms the structural results obtained with NMR studies and is rationalized by a greater stabilization of a larger pocket in horse heart myoglobin relative to sperm whale myoglobin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
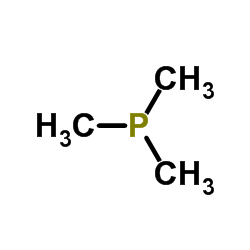 |
Trimethylphosphine
CAS:594-09-2 |
C3H9P |
|
Change of surface structure upon foam film formation.
2015-03-16 [ChemPhysChem 16(4) , 733-8, (2015)] |
|
Molybdenum complex with bulky chelates as a functional model...
2014-12-01 [Inorg. Chem. 53(23) , 12416-27, (2014)] |
|
31P-NMR investigation of trimethylphosphine binding to [alph...
1990-08-31 [Biophys. Chem. 37(1-3) , 407-11, (1990)] |
|
Synthesis and evaluation of technetium-99m monocationic mixe...
1992-08-01 [Int. J. Rad. Appl. Instrum. B 19(6) , 679-84, (1992)] |
|
Orthoacylimines: a new class of chiral auxiliaries for nucle...
2003-04-18 [J. Org. Chem. 68 , 3241, (2003)] |