A method for preparation of amino acid thiohydantoins from free amino acids activated by acetyl chloride for development of protein C-terminal sequencing.
B Mo, J Li, S Liang
Index: Anal. Biochem. 249(2) , 207-11, (1997)
Full Text: HTML
Abstract
A novel and efficient method to prepare amino acid thiohydantoins, which are required as reference standards for development of C-terminal protein sequencing, is reported. Amino acid thiohydantoins were prepared using a straightforward method involving reaction of 20 free amino acids with acetyl chloride as activating reagent and trimethylsilyl isothiocyanate (TMS-ITC) as derivatizing reagent. The products were characterized by HPLC, uv spectra, amino acid analysis, MS, and NMR. Different reaction conditions were investigated and the chemical mechanism of the formation of amino acid thiohydantoins was illustrated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
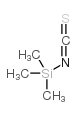 |
trimethylsilyl isothiocyanate
CAS:2290-65-5 |
C4H9NSSi |
|
Multiple pathways in the synthesis of new annelated analogue...
2003-08-21 [Org. Biomol. Chem. 1(16) , 2908-18, (2003)] |
|
Reactivity of fullerene epoxide: preparation of fullerene-fu...
2008-04-04 [J. Org. Chem. 73(7) , 2518-26, (2008)] |
|
Studies in C-terminal sequencing: new reagents for the synth...
1993-04-01 [J. Protein Chem. 12(2) , 195-205, (1993)] |
|
Microsequence analysis of peptides and proteins: trimethylsi...
1987-11-01 [Anal. Biochem. 166(2) , 298-307, (1987)] |
|
Carboxy-terminal sequencing: formation and hydrolysis of C-t...
1990-03-27 [Biochemistry 29(12) , 3145-56, (1990)] |