| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
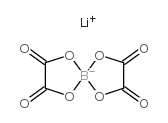 |
Lithium bis(oxalate)borate
CAS:244761-29-3 |
|
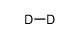 |
deuterium
CAS:7782-39-0 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
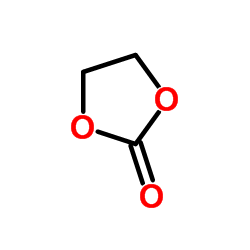 |
Ethylene carbonate
CAS:96-49-1 |
|
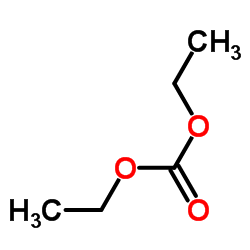 |
Ethyl carbonate
CAS:105-58-8 |
|
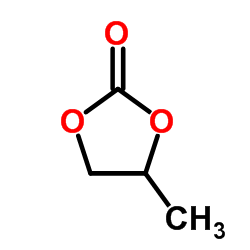 |
Propylene carbonate
CAS:108-32-7 |
|
 |
Cyclosporin A
CAS:59865-13-3 |
|
 |
argon-40
CAS:1290046-39-7 |