The products of 5-fluorouridine by the action of the pseudouridine synthase TruB disfavor one mechanism and suggest another.
Edward J Miracco, Eugene G Mueller
Index: J. Am. Chem. Soc. 133(31) , 11826-9, (2011)
Full Text: HTML
Abstract
The pseudouridine synthase TruB handles 5-fluorouridine in RNA as a substrate, converting it into two isomeric hydrated products. Unexpectedly, the two products differ not in the hydrated pyrimidine ring but in the pentose ring, which is epimerized to arabinose in the minor product. This inversion of stereochemistry at C2' suggests that pseudouridine generation may proceed by a mechanism involving a glycal intermediate or that the previously proposed mechanism involving an acylal intermediate operates but with an added reaction manifold for 5-fluorouridine versus uridine. The arabino product strongly disfavors a mechanism involving a Michael addition to the pyrimidine ring.© 2011 American Chemical Society
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
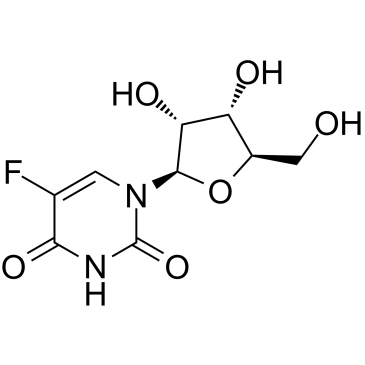 |
5-Fluorouridine
CAS:316-46-1 |
C9H11FN2O6 |
|
Development of an LC-MS/MS assay for the quantitative determ...
2015-06-10 [J. Pharm. Biomed. Anal. 110 , 58-66, (2015)] |
|
N4-[Alkyl-(hydroxyphosphono)phosphonate]-cytidine—New drugs ...
2011-01-01 [Bioorg. Med. Chem. 19(11) , 3520-6, (2011)] |
|
Evidence for Transcriptional Activity in the Syncytiotrophob...
2009-04-01 [Placenta 30(4) , 329-34, (2009)] |
|
Plastid uridine salvage activity is required for photoassimi...
2011-08-01 [Plant Cell 23(8) , 2991-3006, (2011)] |
|
TDP-43 localizes in mRNA transcription and processing sites ...
2009-09-01 [J. Struct. Biol. 167(3) , 235-41, (2009)] |