Oxaceprol is as effective as diclofenac in the therapy of osteoarthritis of the knee and hip.
Shih-Yen Wu, Jia-Wei Kuo, Tien-Kuei Chang, Ren-Shen Liu, Rheun-Chuan Lee, Shyh-Jen Wang, Wuu-Jyh Lin, Hsin-Ell Wang
Index: Clin. Rheumatol. 18(1) , 4-9, (1999)
Full Text: HTML
Abstract
In this multicentre (five centres in Germany), randomised, double-blind, comparative study, 150 patients with painful degenerative joint disease according to EULAR criteria received either oxaceprol (200 mg three times daily) or diclofenac (25 mg three times daily) for 20 days. Joint function, the primary variable, assessed according to Lequesne's indices, improved equally in both treatment groups to a clinically relevant degree. Joint mobility improved by approximately 60% in both groups. By the end of therapy in both groups, the period of pain-free walking time had more than doubled and subjectively evaluated pain perception (VAS) was reduced by almost 50% without any significant differences between the treatments. The incidence of adverse drug reactions was similar in both groups but oxaceprol induced milder symptoms. Oxaceprol is as effective and better tolerated than diclofenac in the treatment of osteoarthritis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
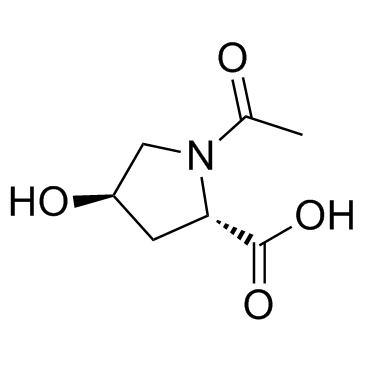 |
Oxaceprol
CAS:33996-33-7 |
C7H11NO4 |
|
Oxaceprol, an atypical inhibitor of inflammation, reduces le...
2001-06-01 [Acta Orthop. Scand. 72(3) , 293-8, (2001)] |
|
Determination of oxaceprol in rat plasma by LC-MS/MS and its...
2011-01-05 [J. Pharm. Biomed. Anal. 54(1) , 173-8, (2011)] |
|
[Assessment of the placebo effect of symptomatic slow-acting...
1998-02-07 [Presse Med. 27(5) , 211-4, (1998)] |
|
Oxaceprol is a well-tolerated therapy for osteoarthritis wit...
2000-01-01 [Clin. Rheumatol. 19(2) , 99-104, (2000)] |
|
Oxaceprol--a randomised, placebo-controlled clinical study i...
2007-01-01 [Clin. Exp. Rheumatol. 25(1) , 29-34, (2007)] |