| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methanol
CAS:67-56-1 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
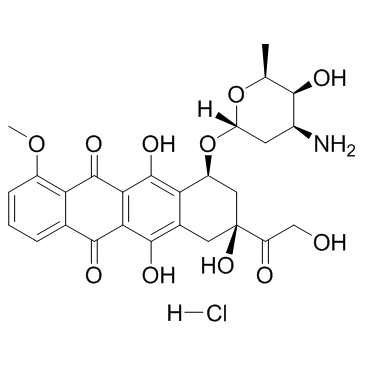 |
Doxorubicin Hydrochloride
CAS:25316-40-9 |
|
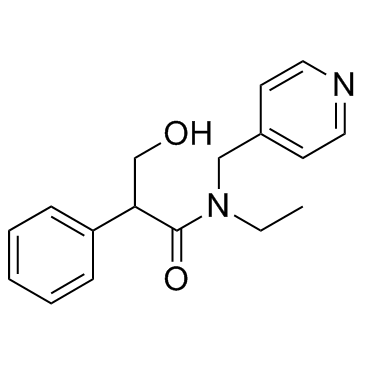 |
Tropicamide
CAS:1508-75-4 |
|
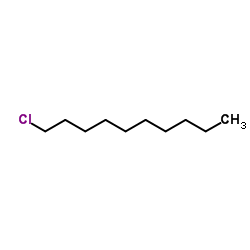 |
1-Chlorodecane
CAS:1002-69-3 |
|
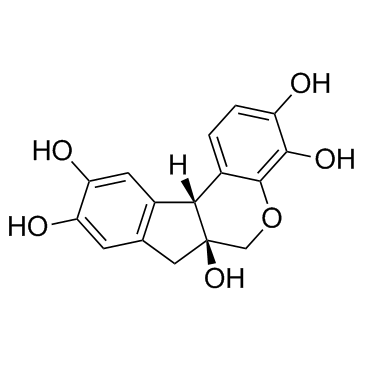 |
Hematoxylin
CAS:517-28-2 |
|
 |
1-Chlorododecane
CAS:112-52-7 |
|
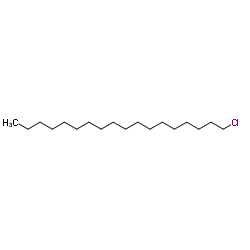 |
1-Chlorooctadecane
CAS:3386-33-2 |
|
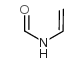 |
N-Vinylformamide
CAS:13162-05-5 |