| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
N-Hexadecyltrimethylammonium chloride
CAS:112-02-7 |
|
 |
Hexadecyl trimethyl ammonium bromide
CAS:57-09-0 |
|
 |
Octyl β-D-glucopyranoside
CAS:29836-26-8 |
|
 |
Thioflavine T
CAS:2390-54-7 |
|
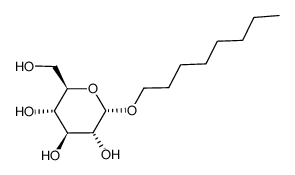 |
N-octyl alpha-D-glucopyranoside
CAS:29781-80-4 |
|
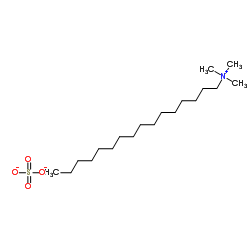 |
hexadecyl-trimethyl-ammonium sulfate
CAS:68214-07-3 |