| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
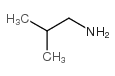 |
Isobutylamine
CAS:78-81-9 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
Piperidine
CAS:110-89-4 |
|
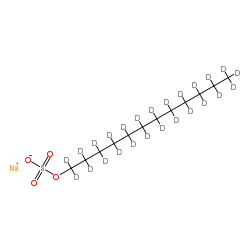 |
Sodium (2H25)dodecyl sulfate
CAS:110863-24-6 |
|
 |
Triisopropylsilane
CAS:6485-79-6 |