Separation of carnitine enantiomers as the 9-anthroylnitrile derivatives and high-performance liquid chromatographic analysis on an ovomucoid-conjugated column.
M Takahashi, K Terashima, M Nishijima, K Kamata
Index: J. Pharm. Biomed. Anal. 14(11) , 1579-84, (1996)
Full Text: HTML
Abstract
9-Anthroylnitrile was used as an achiral reagent for the derivatization of carnitine. The reagent forms UV-absorbing derivatives with the hydroxyl groups of carnitine enantiomers under very mild conditions. The derivatives were separated by high-performance liquid chromatography on an ovomucoid-conjugated column with a mobile phase of acetonitrile-20 mM KH2PO4 (adjusted to pH 4.5 with phosphoric acid) (17:83, v/v). The separation factor (alpha) and resolution (Rs) of the enantiomers were 1.44 and 5.05, respectively. The calibration plots indicated good linearity over a sample concentration ranging from 0.2 to 1.0 mg ml-1, and the detection limit at 254 nm was 0.05 mg ml-1 for each carnitine enantiomer. The reproducibility in the analysis of 1 mg ml-1 of each enantiomer was within 2.0%. The method was applied successfully to the determination of carnitine enantiomers in pharmaceutical preparations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
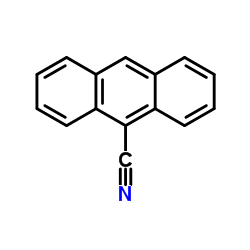 |
9-Cyanoanthracene
CAS:1210-12-4 |
C15H9N |
|
Pendular-state spectroscopy of the S(1)-S(0) electronic tran...
2004-11-15 [J. Chem. Phys. 121(19) , 9489-97, (2004)] |
|
Investigations on the photoreactions of phenothiazine and ph...
2010-09-01 [J. Fluoresc. 20(5) , 1061-8, (2010)] |
|
Simultaneous determination of glucocorticoids in plasma or u...
1998-03-20 [J. Chromatogr. B. Biomed. Sci. Appl. 706(2) , 191-9, (1998)] |
|
Conformational changes in the 23-kilodalton NH2-terminal pep...
1990-11-05 [J. Biol. Chem. 265(31) , 18786-90, (1990)] |
|
Nucleotide-induced movements in the myosin head near the con...
2000-08-31 [Biochim. Biophys. Acta 1481(1) , 55-62, (2000)] |