17beta-Nitro-5alpha-androstan-3alpha-ol and its 3beta-methyl derivative: neurosteroid analogs with potent anticonvulsant and anxiolytic activities.
Scott P Runyon, Matthew Orr, Hernán A Navarro, John A Kepler, Michael A Rogawski, Rafal M Kaminski, C Edgar Cook
Index: Eur. J. Pharmacol. 617(1-3) , 68-73, (2009)
Full Text: HTML
Abstract
Many 17-substituted androstan-3alpha-ol analogs act as positive allosteric modulators of GABA(A) receptors and exert anticonvulsant and anxiolytic-like activity actions in animal models. The endogenous neurosteroid allopregnanolone (17beta-acetyl; 1) is among the most potent of these. Here we demonstrate that 3alpha-hydroxy-17beta-nitro-5alpha-androstane (2b) and its 3beta-methyl analog (3alpha-hydroxy-3beta-methyl-17beta-nitro-5alpha-androstane; 2c) modulate GABA(A) receptors as assessed by [(35)S]t-butylbicyclo-phosphorothionate and [(3)H]flunitrazepam binding with potencies equivalent to or greater than 1. These compounds also had potencies equivalent to or greater than 1 in the pentylenetetrazol and 6Hz seizure models in the mouse. Furthermore, 2b exhibited anxiolytic-like activity in the elevated zero maze. The 3beta-hydroxy, 3alpha-desmethyl analog (2a) was devoid of activity on GABA(A) receptors in vitro but had moderate activity in the seizure models, possibly as a result of epimerization in vivo at the 3-position. This conclusion was supported by the lack of in vivo activity of the 3beta-hydroxy, 3alpha-methyl analog (2d), which is not expected to undergo epimerization. We conclude that nitro can serve as a bioisostere for acetyl at the 17beta-position of 5alpha-androstan-3alpha-ol, such that the nitro analog fully retains the bioactivity of the endogenous neurosteroid at GABA(A) receptors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
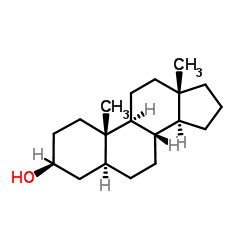 |
5a-androstan-3b-ol
CAS:1224-92-6 |
C19H32O |
|
Impact of induced fit on ligand binding to the androgen rece...
2005-09-08 [J. Med. Chem. 48 , 5666-74, (2005)] |
|
The pheromone androstenol (5 alpha-androst-16-en-3 alpha-ol)...
2006-05-01 [J. Pharmacol. Exp. Ther. 317(2) , 694-703, (2006)] |
|
Synthesis of novel D-ring fused 7'-aryl-androstano[17,16-d][...
2012-04-01 [Steroids 77(5) , 367-74, (2012)] |
|
Sex difference in the proliferative response of mouse hepato...
2003-06-01 [Carcinogenesis 24(6) , 1059-65, (2003)] |
|
Hydrogen bonding between the 17beta-substituent of a neurost...
2009-11-01 [Br. J. Pharmacol. 158(5) , 1322-9, (2009)] |