| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
|p|-Nitrophenyl 2-acetamido-2-deoxy-α-D-galactopyranoside
CAS:23646-68-6 |
|
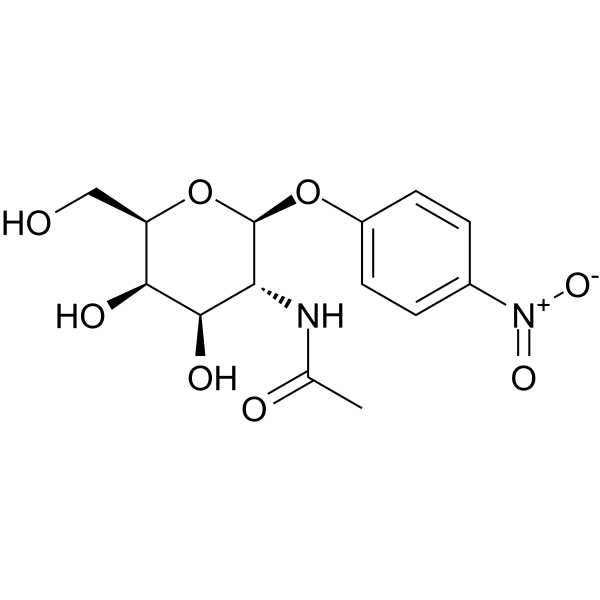 |
4-Nitrophenyl N-acetyl-β-D-galactosaminide
CAS:14948-96-0 |