| Structure | Name/CAS No. | Articles |
|---|---|---|
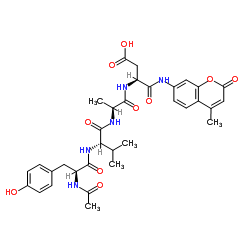 |
Ac-Tyr-Val-Ala-Asp-AMC
CAS:149231-65-2 |
N A Thornberry, H G Bull, J R Calaycay, K T Chapman, A D Howard, M J Kostura, D K Miller, S M Molineaux, J R Weidner, J Aunins
Index: Nature 356 , 768, (1992)
Full Text: HTML
Interleukin-1 beta (IL-1 beta)-converting enzyme cleaves the IL-1 beta precursor to mature IL-1 beta, an important mediator of inflammation. The identification of the enzyme as a unique cysteine protease and the design of potent peptide aldehyde inhibitors are described. Purification and cloning of the complementary DNA indicates that IL-1 beta-converting enzyme is composed of two nonidentical subunits that are derived from a single proenzyme, possibly by autoproteolysis. Selective inhibition of the enzyme in human blood monocytes blocks production of mature IL-1 beta, indicating that it is a potential therapeutic target.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Ac-Tyr-Val-Ala-Asp-AMC
CAS:149231-65-2 |
C33H39N5O10 |
|
Processing/activation of CPP32-like proteases is involved in...
1997-06-01 [Hepatology 25 , 1516, (1997)] |
|
Partial purification and characterization of two distinct ty...
1998-09-01 [J. Invest. Dermatol. 111 , 367-372, (1998)] |
|
Enzymatic activity of two caspases related to interleukin-1b...
1998-04-01 [Eur. J. Biochem. 253 , 76, (1998)] |
|
Multiple intracellular pathways interfere with the activatio...
1998-04-10 [Exp. Cell Res. 240 , 28, (1998)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved