SnCl(4)- and TiCl(4)-catalyzed anomerization of acylated O- and S-glycosides: analysis of factors that lead to higher α:β anomer ratios and reaction rates.
Wayne Pilgrim, Paul V Murphy
Index: J. Org. Chem. 75(20) , 6747-55, (2010)
Full Text: HTML
Abstract
The quantification of factors that influence both rates and stereoselectivity of anomerization reactions catalyzed by SnCl(4) and TiCl(4) and how this has informed the synthesis of α-O- and α-S-glycolipids is discussed. The SnCl(4)-catalyzed anomerization reactions of β-S- and β-O-glycosides of 18 substrates followed first order equilibrium kinetics and k(f) + k(r) values were obtained, where k(f) is the rate constant for the forward reaction (β → α) and k(r) is the rate constant for the reverse reaction (α → β). Comparison of the k(f) + k(r) values showed that reactions of glucuronic acid or galacturonic acid derivatives were ∼10 to 3000 times faster than those of related glucoside and galactopyranoside counterparts and α:β ratios were generally also higher. Stereoelectronic effects contributed from galacto-configured compounds were up to 2-fold faster than those of corresponding glucosides. The introduction of groups, including protecting groups, which are increasingly electron releasing generally led to rate enhancements. The anomerization of S-glycosides was consistently faster than that of corresponding O-glycosides. Reactions were generally faster for reactions with TiCl(4) than those with SnCl(4). Anomeric ratios depended on the Lewis acid, the number equivalents of the Lewis acid, temperature, and substrate. Very high ratios of α-products for both O- and S-glucuronides were observed for reactions promoted by TiCl(4); for these substrates TiCl(4) was superior to SnCl(4). Anomeric ratios from anomerization of S-glucosides were higher with SnCl(4) than with TiCl(4). The dependence of equilibrium ratio on Lewis acid and the number of equivalents of Lewis acid indicated that the equilibrium ratio is determined by a complex of the saccharide residue bound to the Lewis acid and not the free glycoside. The high α:β ratios observed for anomerization of both O- and S-glycuronic acids can be explained by coordination of the C-1 heteroatom and C-6 carbonyl group of the product to the Lewis acid, which would enhance the anomeric effect by increasing the electron-withdrawing ability of the anomeric substituent and lead to an increase in the proportion of the α-anomer. Such an observation would argue against the existence of a reverse anomeric effect. Support for a chelation-induced endocyclic cleavage mechanism for the anomerization is provided by the trapping of a key intermediate. The data herein will help predict the tendency of β-glycosides to undergo anomerization; this includes cases where 1,2-trans glycosides are initial products of glycosidation reactions catalyzed by TiCl(4) or SnCl(4).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Titanium tetrachloride
CAS:7550-45-0 |
Cl4Ti | |
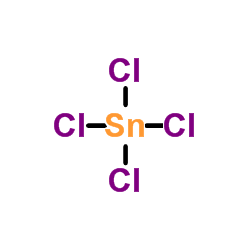 |
Tin(IV) chloride
CAS:7646-78-8 |
Cl4Sn |
|
SPREDs (Sprouty related proteins with EVH1 domain) promote s...
2015-04-01 [Dev. Dyn. 244(4) , 591-606, (2015)] |
|
Is sexual reproduction of high-mountain plants endangered by...
2015-04-01 [Oecologia 177(4) , 1195-210, (2015)] |
|
Comparison of a novel TiO₂/diatomite composite and pure TiO₂...
2014-06-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 960 , 52-8, (2014)] |
|
Quantitative distribution of Salmonella spp. and Escherichia...
2015-10-01 [Int. J. Food Microbiol. 210 , 149-55, (2015)] |
|
ISG15 regulates RANKL-induced osteoclastogenic differentiati...
2015-01-01 [Biol. Pharm. Bull. 38(3) , 482-6, (2015)] |