Biosynthesis of N-acetyldopamine and N-acetyloctopamine by Schistocerca gregaria nervous tissue.
A K Mir, P F Vaughan
Index: J. Neurochem. 36(2) , 441-6, (1981)
Full Text: HTML
Abstract
N-Acetyltyramine, N-acetyldopamine and N-acetyloctopamine were the major products when either L-[3H]tyrosine or [3H]tyramine were incubated with thoracic ganglia of the desert locust, Schistocerca gregaria. No label was incorporated into L-DOPA under these conditions, although 2-3% of the radioactivity could be recovered in dopamine and octopamine. Addition of the aromatic amino acid decarboxylase inhibitor, 3-hydroxybenzylhydrazine (NSD 1015), prevented the formation of N-acetylcompounds from L-[3H]tyrosine, without resulting in an accumulation of label in L-DOPA. In contrast, incubation of samples of haemolymph with L-[3H]tyrosine resulted in the recovery of 7% of label in L-DOPA, which was increased to 17% in the presence of NSD 1015. These results provide evidence that the initial step in the synthesis of dopamine and octopamine by S. gregaria nervous tissue is the conversion of L-tyrosine to tyramine, which is subsequently metabolised to N-acetyltyramine, N-acetyldopamine or N-acetyloctopamine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
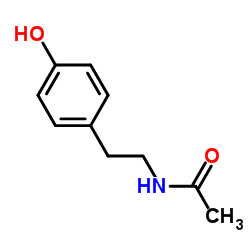 |
N-Acetyltyramine
CAS:1202-66-0 |
C10H13NO2 |
|
Selective nanomolar detection of dopamine using a boron-dope...
2009-05-15 [Anal. Chem. 81(10) , 4089-98, (2009)] |
|
Mechanistic and structural analysis of Drosophila melanogast...
2014-12-16 [Biochemistry 53(49) , 7777-93, (2014)] |
|
Effect of acetyl derivatives of some sympathomimetic amines ...
1977-02-01 [Acta Pharmacol. Toxicol. (Copenh.) 40(2) , 247-58, (1977)] |
|
Partial purification and properties of N-acetylhistamine dea...
1976-07-08 [Biochim. Biophys. Acta 438(2) , 532-9, (1976)] |
|
Effect of spores of saprophytic fungi on phytoalexin accumul...
2000-08-01 [J. Agric. Food Chem. 48(8) , 3662-5, (2000)] |